Corindus
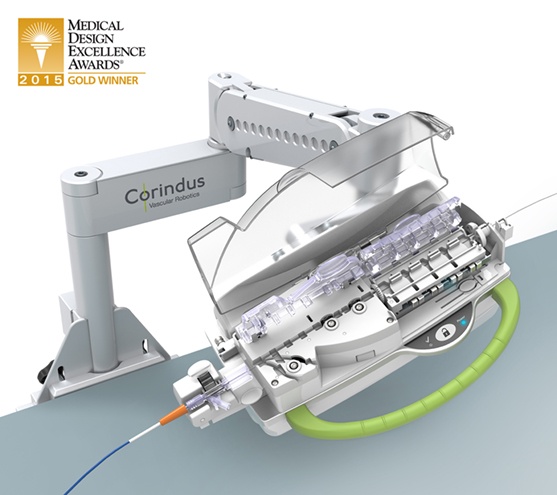
CorPath 200 System
Corindus, a startup company working in the field of catheter lab robotics, challenged Farm to develop and refine their existing percutaneous coronary intervention (PCI) system; used by physicians to robotically control and deliver guidewires and other angioplasty devices to a target lesion deep inside a patient’s vascular system with enhanced precision and safety. The system is comprised of a robotically-driven, single-use cassette, robotic drive base, articulating positioning arm, and operator control console. Farm was tasked with completely redesigning the Corindus system to improve its performance, reliability and manufacturability. The result is the CorPath 200 System.
Farm led the systems engineering effort for development of Corpath, including technology and requirements development; human factors, mechanical engineering, software and electronics design. In order to ensure wide clinical acceptance, an open architecture system was developed to support the dozens of guidewire, balloon and stent products currently manufactured by industry leaders.Developing intellectual property to secure competitive advantage in the market was essential for Corindus. To help our client achieve this goal, Farm developed proprietary and patentable technologies for driving and rotating coronary guidewires and devices, converted the durable cassette to single use, and engineered the interface of the cassette to the robotic drive base. To optimize access to the patient and ensure articulation and maneuverability of the catheter inside the body, our Human Factors team determined the impact of varying patient sizes to inform the articulating arm’s architecture and required range of motion. The guidewire and device loading process was simplified for users by minimizing steps and allowing easy identification of interaction points including levers and controls. One of the key benefits of the new system is that the physician no longer needs to wear a cumbersome lead apron to protect them from the X-rays that are used during a procedure to “see” inside the patient. When using the Corpath 200, the physician is able to perform PCI procedures while sitting within a lead-shielded control cockpit. The control console was designed to optimize the footprint inside an already crowded catheter lab, and includes an angled touch-screen, joystick and easy to navigate graphical user interface to minimizes head, neck, and back strain.
Concurrent to the specification and technology development, our team undertook the effort to commercialize the system. Working under Farm’s ISO 13485-certified development controls, we delivered a reliable system architecture; mechanical, software and electronic sub-systems (including a custom, Windows-based single-board computer); single-use cassette design optimized for manufacturability; and fully functioning, pre-clinical prototype devices.
The Corindus CorPath 200 is the only robotic catheterization system to have received FDA 510(k) clearance for use in PCI procedures. In late 2015, they received a clearance for radial access procedures, and more recently, won FDA 510(k) clearance for the CorPath system to be used in peripheral interventions, a year after launching a clinical trial that studied the safety and effectiveness of the CorPath device in peripheral artery disease patients with lower-extremity blockages.
Additional Information: