We understand different customer segments have different needs, different jobs to complete, and different challenges to overcome. Our human factors experts are involved at each step of the development process, from early user needs definition through summative usability validation, to ensure we meet the disparate needs of distinct user groups. We have hands-on, global experience with thousands of in-field observations and in-depth interviews and believe a user insight is more than an observation – it’s an actionable design opportunity that can differentiate your brand.
Creating world-class healthcare systems is an iterative process requiring close collaboration between all healthcare stakeholders. We evaluate our solutions with novices and experts alike throughout the process, using state-of-the-art usability practices tailored to specific healthcare domains. So, whether your designing a new wearable drug delivery device, a cardiovascular imaging system, or a complex surgical robot, we have the knowledge and the tools to evaluate your product for safety, for usability, and for the user experience.
We know medical.
We understand the challenges of developing a new product or service in a regulated industry, and we’re acutely aware of the safety risks associated with complex medical devices. Our human factors team members are experts in mitigating safety hazards and documenting the process to meet regulatory standards.
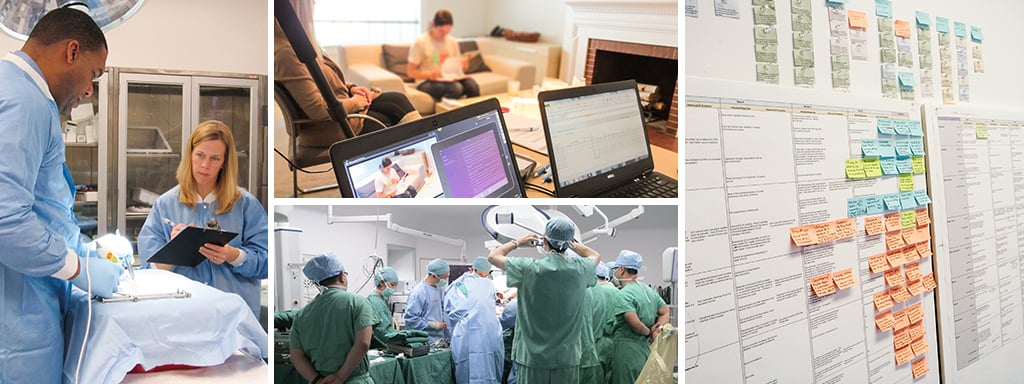
Product design begins with a deep understanding of the potential end users, the intended use environment, and scenarios of use. We immerse ourselves into these areas to generate relevant inputs to design.
We know that good products result from an integrated user-centered design approach, so we iteratively evaluate safety, usability, and user satisfaction throughout the development process.
We help you evaluate whether critical use-related risks are adequately mitigated, ensuring design goals have been met.
With hundreds of approved medical products on the market, we’re here to help you navigate the regulatory pathway to ensure your product can go to market, anywhere in the world, with confidence.
We collaborate with clients to ensure the human factors portion of the design history file is complete.