The FDA recently released the latest version of three guidance documents addressing unique device identifiers, adaptive study designs, and real world evidence in regulatory research. In addition, two guidance documents related to whether a 510(k) is needed when making changes to an existing or pre-amendment device were released. To get a better sense of what these guidance documents will mean for medical device product development, we took a closer look at each, and have provided our insights below.
Adaptive Design for Medical Device Clinical Studies
Adaptive studies involve a prospective (i.e., a priori) alteration to the clinical study protocol, and often require careful planning in the design phase. This guidance relates most strongly to feasibility studies or pivotal clinical trials, and provides important suggestions and parameters for the conduct of such studies to maintain proper empirical and ethical boundaries during the course of research.
At the core of adaptive design are two principles: plan ahead, and be transparent. All plans to change the study protocol, whether the change pertains to sample size, longevity, or analysis, need to be justifiable for the benefit of the study and, most importantly, the benefit of the patients and end users of the device being tested.
Unique Device Identification System: Form and Content of the Unique Device Identifier (UDI)
In September of 2013, The FDA came out with the Unique Device Identifier (UDI) rule, which states that any medical device used and marketed in the United States must have a UDI, which an FDA-accredited facility would provide.
The UDI must be present in two forms: plain text as well as automatic identification and data capture (AIDC) technology (i.e. barcode or similar technologies). The plain text is present so that health care providers, patients, FDA members, and other device users accurately record and enter the UDI as data when needed. AIDC technology, on the other hand, allows for fast and accurate data communication and record keeping within and across facilities.
The guidance itself is short and to-the-point. It totals ten pages, and provides instructions as to acceptable sources and technologies needed to obtain a UDI for your product.
Use of Real-World Evidence to Support Regulatory Decision Making for Medical Devices
This draft guidance is meant to supplement and support the regulations already in place for evidentiary support in medical device approval applications. It details the types of real-world data (RWD) that have the potential to inform the FDA of device use patterns and efficacy. Examples of RWD might include administrative and claims data, quality improvement registries, and electronic health records (EHRs), and it can be helpful when sponsors are looking to conduct postmarket surveillance, expand indications for use, carry out post-approval surveillance commitments, identify a control group, etc.
Still, the guidance emphasizes that the consideration of this type of data is varied, and its acceptance depends heavily on the extent and quality of data that sponsors are able to obtain. Some sources of RWD might be appropriate for postmarket surveillance only, whereas others might also be able to inform premarket decisions regarding product safety.
The inclusion of high quality (reliable across collection sites and/or over time) RWD as evidence in both pre- and postmarket development can be a cost savings and might prove useful for those products which significantly benefit patients, therefore, it should be distributed as soon as possible. It is important to keep in mind, however, that RWD could still fall under investigational device exemption (IDE) regulations, and whether or not IDE regulations apply would be determined on a case-by-case basis.
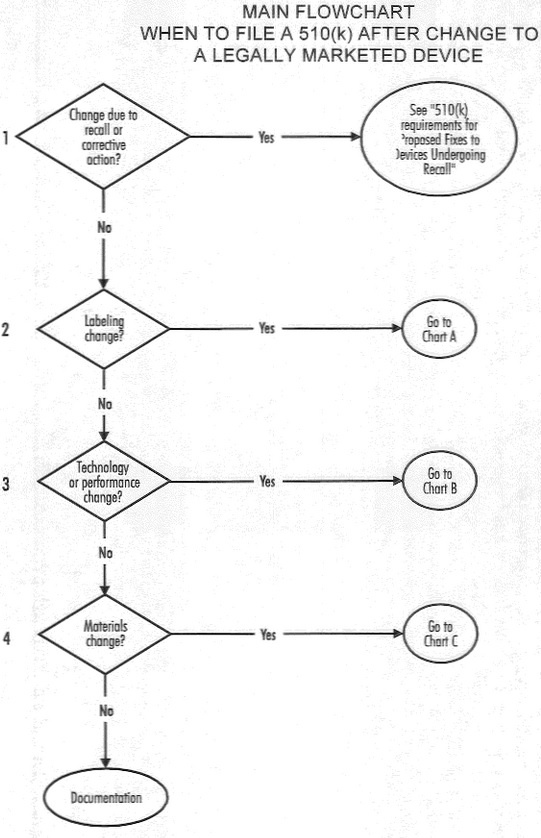
510(k) Submission for Existing and Pre-amendment Devices
The FDA also recently released two guidance documents to help sponsors and manufacturers determine whether a new premarket notification or 510(k) is needed when making changes to an existing or pre-amendments device. One is for devices, generally, and the other is for software products. There are a series of very helpful flow charts within the guidance documents that help manufacturers determine the need for a new 501(k). Ultimately, however, the FDA asserts that they will make decisions on a case-by-case basis. The dynamic nature of the medical device field makes it difficult to create hard and fast rules that can apply to a diverse set of devices. These guidance documents do their best to convey the types of changes that will require a 510(k) by emphasizing that, at its core, a 510(k) is required when changes to the device significantly affect the safety and risk associated with proper use of the device. This might seem somewhat vague, but it can be applied to everything from labeling to material changes. When making the decision as to whether or not your device will require a new 510(k), it’s best to refer to the flow charts provided or communicate directly with the FDA.